Orforglipron: the Weight-Loss Pill to Watch – What We Know Now
If you’ve been waiting for a pill instead of a weekly shot, orforglipron may be the candidate on your radar. It’s an oral GLP-1 receptor agonist – a class known for appetite and weight effects – taken once a day. The idea is simple: keep the biology of GLP-1, lose the needles. Reality is messier, as always, but the evidence is building.
What is orforglipron, in plain terms?
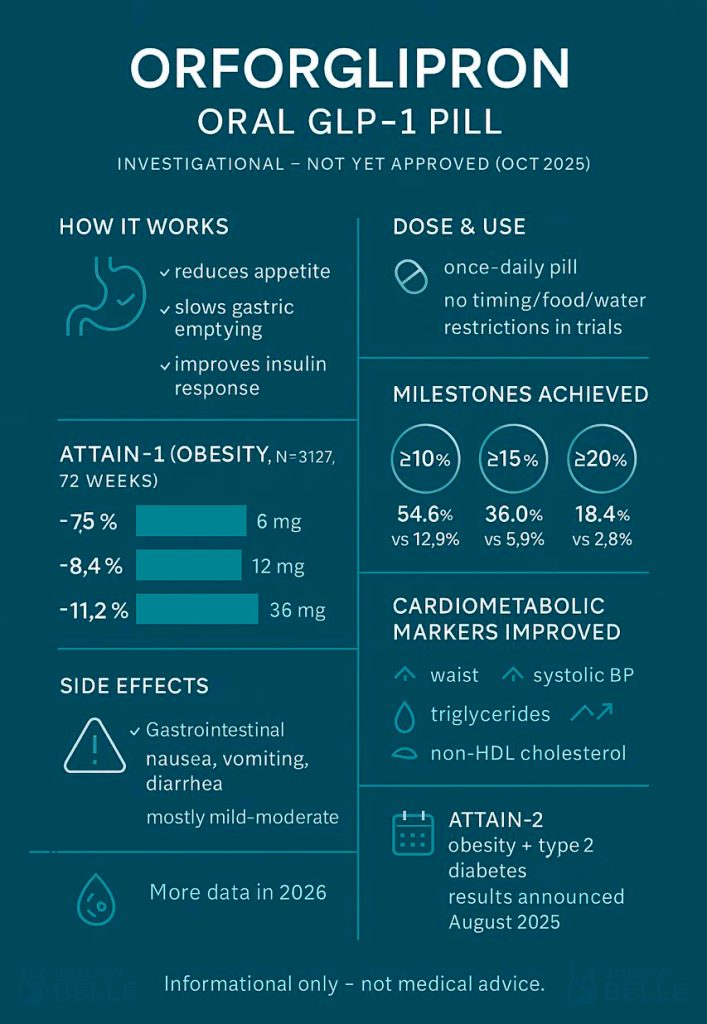
It’s a small-molecule, non-peptide GLP-1 receptor agonist designed for type 2 diabetes and for obesity treatment. Unlike peptide GLP-1s you inject, this comes as a tablet. In trials it was dosed once daily – without specific timing, food, or water restrictions – which, honestly, is the draw for many of you who dislike injections or complex dosing rules. It’s still investigational; no approvals yet anywhere.
How much weight loss are we talking about?
ATTAIN-1, a 72-week Phase 3 trial in adults with obesity but without diabetes (n≈3,100), reported average weight reductions of roughly 7.5% with 6 mg, 8.4% with 12 mg, and 11.2% with 36 mg, versus ~2.1% for placebo. More people on the highest dose hit the bigger milestones too: ≥10%, ≥15%, and ≥20% body-weight loss were all notably more common than with placebo. If you’re doing the mental math, a 10% drop is often the clinical “line in the sand” for risk reduction – and many participants crossed it.
Cardiometabolic picture – not just the scale
Beyond weight, markers moved in the right direction: waist circumference, systolic blood pressure, triglycerides, and non-HDL cholesterol improved more with orforglipron than with placebo. Possibly that’s the bigger story long-term, because risk is what we’re trying to bend. Still, results vary, and your baseline risk matters.
Safety and tolerability – the part everyone skims (please don’t)
The most common side effects were gastrointestinal – think nausea, vomiting, diarrhea – usually mild to moderate. Discontinuations due to adverse events happened in roughly 5–10% on orforglipron versus ~3% on placebo. If you’ve followed GLP-1s, that profile will look familiar. It’s not a free lunch – and if your gut is sensitive, you’ll want a candid conversation with your clinician.
Where things stand with regulators
As of mid-October 2025, orforglipron is not approved. Lilly says it plans to file for obesity in 2025 and for type 2 diabetes in 2026, with availability depending on how reviews go. There’s industry chatter about the FDA’s new fast-track voucher program, but the company has been cautious in public – so don’t bank on shortcuts. Possibly fast, possibly not. Patience helps.
How it stacks up against other treatments
Two diabetes-focused Phase 3 readouts this October matter for the broader story. In one trial, orforglipron cut A1C by up to ~1.7% and outperformed dapagliflozin (an SGLT2 inhibitor). In another, adding orforglipron to insulin glargine drove A1C down even further. Weight also moved in the right direction. That’s diabetes data, not an obesity head-to-head, but it suggests metabolic potency consistent with the class. A separate head-to-head against oral semaglutide in type 2 diabetes was also positive for orforglipron. The pill looks competitive – with the usual caveats about different populations and endpoints.
What we know so far
- Orforglipron is a once-daily oral GLP-1 RA, studied for obesity and type 2 diabetes; not approved yet.
- In ATTAIN-1 (72 weeks), mean weight loss ranged about 7.5%–11.2% across doses vs ~2.1% with placebo, with meaningful cardiometabolic gains.
- GI effects are common; discontinuations were ~5–10% on drug vs ~3% on placebo.
- Regulatory filings for obesity are planned for 2025; diabetes filings expected in 2026.
What we don’t know (yet)
- Real-world adherence when the novelty fades – pills can be “easier,” but daily routines fail.
- How it compares directly with leading injectables for obesity over 1–2 years – we need head-to-head obesity trials, not just diabetes readouts.
- Long-term safety beyond the current dataset – larger, longer studies and post-approval surveillance will tell us more.
Who might consider it if approved
- You want GLP-1-like effects but prefer a pill over injections.
- You’ve had access or supply issues with injectables and need another route.
- You have obesity-related risks where a 10% weight loss could change your risk profile – and you’re prepared for GI trade-offs.
Who might pass (or wait)
- You’ve had tough GI reactions to GLP-1s and aren’t ready to try again.
- You’re chasing 15–20% weight loss and already respond well to injectables – you might want to see more head-to-head obesity data first.
- You need coverage now – and can’t sit through regulatory timelines.
Our take
If you’re looking for magic, you’ll be disappointed. If you’re looking for options, this is real progress. A daily pill that delivers steady, double-digit weight loss for a good chunk of users is not a small thing. It lowers friction – no pens, no needles – and that can mean persistence. Possibly that’s the quiet superpower here. But – and here comes the sharp edge – tolerability still matters, expectations still matter, and behavior still matters. A pill won’t rewrite physics. It can tilt the odds.

Practical notes if you’re planning ahead
Should orforglipron get the green light, talk to your clinician about dose, titration, and how to navigate GI effects. Plan for the basics: protein intake, fiber, fluids, and a movement routine you can stand. Keep labs on schedule. If side effects bite, don’t ghost your care team – adjust. Honestly, that’s where people win or lose with GLP-1s.
Bottom line
Orforglipron is a serious, once-daily oral contender in the GLP-1 era. The weight-loss and cardiometabolic data are credible; the convenience is obvious; the safety looks class-consistent. Approval isn’t guaranteed, but the trajectory is clear. If you want a needle-free path with proven biology, keep this on your shortlist – eyes open, expectations steady.
